
 |
 |
| |
| |
Linear Polarisation Resistance Measurement.
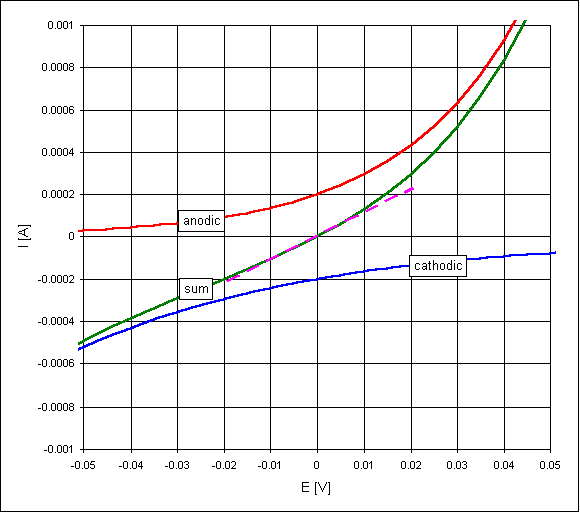
The voltage-current response of a corroding electrode tends to be linear over a small range of potential either side of the free corrosion potential. This is because both the anodic and cathodic currents are exponentially related to potential, the difference between two such exponential curves is nearly linear over a small range of potential.
This was first noted in the now famous paper by Stern and Geary. As the plot below shows, even for sensible values of the Tafel slopes (60 and 120 mV), the linearity leaves a lot to be desired. The slope of the response at the corrosion potential, the polarisation resistance, is then inversely proportional to the corrosion current.
In theory the proportionality constant can be calculated from the Tafel slope values - in practice an empirically derived 'fiddle factor' is commonly used.
There are a number of ways of carrying out the LPR measurement. Perhaps the simplest method is to use two nominally identical electrodes. A small (say 20 mV) potential difference is applied between these and the resulting current is measured. This is then proportional to the inverse of the polarisation resistance and hence is directly proportional to the corrosion rate.
A more sophisticated approach is to use a potentiostat and a three electrode arrangement. The test electrode is polarised by a small amount (say 10 to 20 mV) from its free corrosion potential and the required current is measured. Again, this is directly proportional to the corrosion rate.
Problems arise when the response is non-linear or is linear over only a very small range of potential. The actual method of application of the perturbation - for example a potential step, a repetitive waveform or a slow linear sweep - can also greatly affect the measurement. Electrochemical noise can make reliable measurements difficult. And, as with all these perturbative techniques, the method effectively averages over the whole of the test electrode - localised corrosion attack can be hard to detect.
Some of these problems are overcome by the a.c. impedance measurement technique.